こばし鍼灸(掃骨)院 の日記
-
###直原稿 編集中
2019.03.15
-
ORIGINAL COMMUNICATION
Dermatome and Fasciatome
CARLA STECCO ,1* CARMELO PIRRI,2 CATERINA FEDE,1 CHENGLEI FAN,1
FEDERICO GIORDANI,3 LUIGI STECCO,4 CALOGERO FOTI,2
AND RAFFAELE DE CARO 1
1Department of Neuroscience, Institute of Human Anatomy, University of Padova, Italy
2Physical and Rehabilitation Medicine, University of Rome “Tor Vergata”, Rome, Italy
3Physical and Rehabilitation Medicine, University of Padova, Padova, Italy
4Fascial Manipulation Institute, Padova, Italy
Increased knowledge of the rich innervation of the deep fascia and its anatomical
organization indicates the need to reevaluate maps of the dermatome according
to the new findings. The authors present a distinction between dermatome and
fasciatome, basing their approach to the literature on nerve root stimulation and
comparing dermatomeric and myomeric maps. The former represents the portion
of tissue composed of skin, hypodermis, and superficial fascia supplied by all the
cutaneous branches of an individual spinal nerve; the latter includes the portion of
deep fascia supplied by the same nerve root and organized according to force lines
to emphasize the main directions of movement. The dermatome is important for
esteroception, whereas the fasciatome is important for proprioception. If they are
altered, the dermatome shows clearly localized pain and the fasciatome irradiating
pain according to the organization of the fascial anatomy. Clin. Anat. 00:000–000,
2019. © 2019 Wiley Periodicals, Inc.
Key words: fascia; dermatome; nerve; pain; proprioception
INTRODUCTION
The textbooks now commonly used in medical and
allied health programs contain multiple, conflicting dermatome
maps (Ladak et al., 2014). The consequence
for clinical practice is confusion in evaluating radiating
pain. A “dermatome” is typically defined as the region
of skin supplied by all cutaneous branches of a single
spinal nerve (Kishner et al., 2017): it must be distinguished
from the “myotome,” which is the group of
muscles innervated by a single spinal nerve, and from
the “sclerotome,” a region of bone and periosteum
innervated by a single spinal segment (Inman and
Saunders, 1944). The three maps do not overlap and,
more importantly, they show completely different patterns,
mainly in limbs.
Initial research to determine the extent of each dermatome
was carried out in Europe during the late 19th
and early 20th centuries. The first account of the distribution
of segmental nerve fibers of the upper limbs was
published in 1886 by Sir Wilmot Herringham, based on
the results of his dissections of neonatal and adult
cadavers (Herringham, 1886). Sir Henry Head was the
first to produce a dermatome map based on clinical
observation of referred visceral pain and traumatic
lesions of the spinal cord (Head, 1893). During the late
19th century, Sir Charles Sherrington studied this subject
further, using rhesus monkeys and severing the
dorsal nerve roots above and below the nerve studied
(Sherrington, 1898). A similar approach was also used
by Otfrid Foerster to define the dermatomes of
the lower limbs in humans (Foerster, 1933). In 1948,
Keegan and Garrett published a radically different map,
which has been reproduced in many textbooks (Keegan
and Garrett, 1948). More recently, Denny-Brown et al.
significantly altered the traditional view of a static dermatome
map, in which the size of the dermatome
*Correspondence to: Carla Stecco, Section of Anatomy, Department
of Neuroscience, University of Padova, Via A. Gabelli 65, 35121
Padova, Italy. E-mail: carla.stecco@unipd.it
Received 22 March 2019; Revised 29 April 2019; Accepted 9
May 2019
Published online 00 Month 2019 in Wiley Online Library
(wileyonlinelibrary.com). DOI: 10.1002/ca.23408
© 2019 Wiley Periodicals, Inc.
Clinical Anatomy (2019)
changes according to the characteristics of adjacent
spinal cord segments, indicating that the dermatome is
in fact dynamic and dependent on central communications
among spinal levels (Denny-Brown et al., 1973;
Denny-Brown and Kirk, 1968; Kirk and Denny-Brown,
1970). Lee et al. (2008) constructed a new map based
on clinical reports and studies of nerve block and
peripheral nerve stimulation.
The current state of our knowledge indicates many
discrepancies in the relevant literature, which hinders
applications in clinical practice and causes difficulties for
students (Challoumas et al., 2018). According to recent
studies on deep fascia innervation, one explanation for
all these different maps is that no study has distinguished
the innervation of the skin fromthat of the deep
fascia. However, we now know that the deep fascia is
very well innervated (Hoheisel et al., 2011; Stecco
et al., 2008) and that it could be a source of pain irradiation
with different patterns from the skin (Schilder et al.,
2014). Willard et al. (2012) introduced the term
“fasciotome” to describe the specific innervation of the
thoracolumbar fascia, according to the difference in
innervation between the thoracolumbar fascia and the
skin of the back. On the basis of that description, the
present study reviews the literature on fascial innervation
in order to ascertain whether the deep fascia can be
innervated differently from the overlying skin and consequently
have its ownmap of pain distribution.
METHODS
This article is not intended to be a comprehensive
article, but rather a commentary review of published
articles containing the terms “innervation,” “fascia,”
“superficial fascia,” or “deep fascia” in their titles. The
PubMed database was searched for clinical studies with
these key terms. Our research involved combining
these terms using the Boolean operator “AND.” It covered
case reports, clinical trials, controlled clinical trials,
reviews, comparative studies, multicenter studies, and
randomized controlled trials in humans and other animals.
Our search was expanded using the reference lists
in these texts. Important secondary references were
also used. Studies in English in which the word “fascia”
is connected with “innervation” were examined; all
other articles were excluded from the present review. A
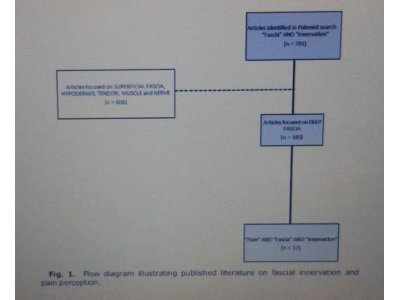
PubMed search for “innervation and fascia” yielded
791 articles. This number was reduced by eliminating
606 works on superficial fascia, subcutaneous tissue,
hypodermis, nerves, tendons, and muscles. The remaining
articles, indicating “innervation and deep
fascia,” totaled 185. When another search key word,
“Pain,” was added, the succeeding search for “Pain AND
innervation AND deep fascia” yielded 37 papers (Fig. 1).
As a template for spinal nerve sensory distributions
and peripheral nerve territories, we examined dermatome
and myotome maps of the upper and lower limbs
Fig. 1. Flow diagram illustrating published literature on fascial innervation and
pain perception.
2 Stecco et al.
in the 41st edition of Gray’s Anatomy (Standring,
2016) and also those described by Ladak et al. (2014),
Furman and Stephen (2019), Slipman et al. (1998),
and Schirmer et al. (2011).
RESULTS
Fascial Anatomy
The Terminologia Anatomica defines “fascia” as a
sheath, a sheet, or any number of other dissectible
aggregations of connective tissue. Consequently, two
types of fascia are distinguished: the superficial fascia,
which is connected to the skin, and the deep fascia connected
by fibrous septa (retinaculum cutis superficialis
and profundus, respectively), which impart specific
mechanical properties to the subcutis (Nash et al.,
2004). The two kinds of retinaculum cutis differ considerably
(Lancerotto et al., 2011). The deep septa are rare,
thin, and oblique, allowing great autonomy between
superficial and deep fasciae. In contrast, the superficial
septa are short, vertically oriented, and dense, connecting
the superficial fascia to the skin (Stecco, 2015).
The deep muscular fascia is a fibrous layer that
envelops not only all the muscles but also tendons,
joints, and ligaments, connecting several elements of
the musculoskeletal system and transmitting muscular
force over a distance (Stecco, 2015). It can sense the
basal tone of the underlying muscles because of its
many muscular and tendinous insertions (Fig. 2); all
these connections are called myofascial expansions.
Marshall (2001) estimated that only 70% of muscular
Fig. 2. (a), Schema representing myofascial expansions of anterior muscles of
upper limb in which the continuity along the movement line of anteposition or flexion is
highlighted. Note that pectoralis major, biceps brachii, flexor carpi radialis, and flexor
pollicis brevis muscles have myofascial expansions into the brachial fascia, which is consequently
tensioned each time the upper limb moves in an anterior direction. (b),
Myofascial expansion of semitendinosus muscle into deep fascia. (c), Schema representing
myofascial continuity along the line of movement of lateroposition or abduction
allowed by the superficial fibers of gluteus maximus and tensor fasciae latae
muscles, iliotibial tract, lateral parts of biceps femoris and vastus lateralis, and fibularis
muscles. Along the line of retroposition or extension, the fascia is stretched by the deep
fibers of gluteus maximus muscle, by ischiocrural and gastrocnemius muscles, tomerge
into plantar fascia. (d), Relative percentages of bone and fascial insertions of muscle,
according to Marshall (2001). [Color figure can be viewed at wileyonlinelibrary.com]
Dermatome and Fasciatome 3
forces are transmitted to the bones to perform movements;
30% are transmitted to the fascial components
around the muscles. Thus, every time the muscles
contract, they produce tension in the fascia and this
mechanical input can create specific fibrous reinforcements
day by day, visible macroscopically during
dissection. Thanks to these myofascial connections,
anatomical continuity is created among various muscles
involved in the same directional movement, challenging
the classical concept of muscles as morphologically
independent actuators. Wilke et al. (2016) assigned a
clinical application to these continuities along the body,
demonstrating that the tension of the myofascial elements
in the posterior region of the lower limbs can
affect the range of motion (ROM) of the neck and that
the consequent stretching of the ischio-crural muscles
can improve that ROM.
Myofascial expansions are always present and
show precise spatial orientation. In particular, they
stretch the aponeurotic fasciae of the limbs along
the six main directions of movement (Stecco et al.,
2008): anteposition, retroposition, adduction, abduction,
intrarotation, and extrarotation. We prefer the
terms anteposition and retroposition to flexion and
extension, because the hip and knee—for example, during
a kick—perform both anteposition and stretching of
the anterior portion of the fascia lata and crural fascia.
According to the classical definition of such movements,
the hip is flexed and the knee is extended, which seem
to be opposite movements.
Innervation of the Fascia
Recent research shows that the deep fascia is richly
innervated (Stecco et al., 2007; Taguchi et al., 2013;
Tesarz et al., 2011) and could be active in proprioception
and the perception of pain. The nerve fibers in the deep
fascia can be either peptidergic or non-peptidergic.
Taguchi et al. (2013) showed that the free nerve endings
are both Aδ and C types. Aδ fibers appear to be sensitive
mainly to mechanical stimuli such as clamping, whereas
most C-type fibers are polymodal (nociceptors) and
therefore sensitive to both mechanical and chemical
stimuli (e.g., bradykinin) and to heat. In addition, C
fibers in the deep fascia have a very high mechanical
activation threshold (1,854 mN), about twice that of skin
or muscle. Schilder et al. (2014) found that stimulation
of the thoracolumbar fascia in healthy volunteers with
hypertonic saline can generate pain, which is more
intense and has greater irradiation than the same solution
causes when injected into the muscular mass of the
erector spinae. Similar results were obtained by Deising
et al. (2012) with injections of nerve growth factor into
the thoracolumbar fascia. Schilder et al. (2018) concluded
that electrical stimulation of various soft tissues
in the lower back reveals distinct pain quality patterns
for muscles versus fascia and skin: the features of “deep
pain” point toward muscle as the relevant target,
whereas “heat pain” or “sharp pain” indicates the fascia.
Schilder et al. (2018) also stated that the descriptor patterns
of fascia and skin can lead to misinterpretation of
fascia-related pain in the lower back pain as neuropathic.
They also observed long-term sensitization of deep fascia
nociceptors to mechanical pressure and chemical
stimulation with acids. This mechanism could explain
chronic musculoskeletal pain. In addition, the same
authors showed that the free nerve endings of the fascia
are stimulated most effectively when the fascia is “prestretched”
by muscle contraction. Electrical stimulation
of the deep fascia produces a dull, annoying pain,
whereas the same stimulation of the hypodermis and
superficial fascia produces acute and clearly localized
pain (Itoh et al., 2004). This suggests that the two types
of fascia have different roles: the deep fascia has a
mainly proprioceptive function, whereas the superficial
fascia cooperates with the skin for esteroception. The
interposed adipose layer between the fasciae (DAT =
deep adipose tissue) probably works by insulation, allowing
the two fasciae to flow and be stretched independently.
We suggest that the DAT should be viewed as the
“watershed” between the exteroceptive system (formed
of skin, superficial adipose tissue, and superficial fasciae)
and the proprioceptive system (located in muscles
and deep fasciae). Where the DAT disappears and the
superficial and deep fasciae fuse (as in the palm of the
hand and the plantar part of the foot), the esteroceptive
and proprioceptive systems are combined. This facilitates
the perception of form, volume, and the surfaces
of various objects, and consequently movement, guaranteeing
adaptation of the foot and hand to various
contact surfaces. Anatomical variations are clearly
recognizable and, predictably, dermatomal maps differ
among individuals.
Taguchi et al. (2008) reported that the sensory endings
project to spinal cord areas located in the dorsal
horn, two to three segments cranially relative to
the location of the terminal endings. This innervation
pattern appears congruent with the underlying musculature.
Chronic irritation of the muscle fascia can also
induce sensitization at spinal level. Hoheisel et al.
(2011) reported that the metamers affected by nociceptive
afference increased in rats with chronic inflammation
of the thoracolumbar fascia, and Taguchi et al.
(2013) showed that repeated mechanical stimuli can
stimulate the expression of c-Fos protein in the spinal
metamers to which the sensitive fibers belong. Gibson
et al. (2009) showed that the deep fascia—and not
the muscle—is probably responsible for sensitization
and/or pain associated with delayed onset muscle soreness
following eccentric exercises. Hoheisel et al.
(2015) reported an increase in SP-positive fibers (nociceptive)
in a chronically inflamed thoracolumbar fascia,
showing that the fascia can undergo pathological
changes leading to long-term worsening of symptoms.
Similar data were published by Sanchis-Alfonso and
Rosellò-Sastre (2000) concerning the level of the lateral
retinaculum of the knee, reporting growth of nociceptive,
immunoreactive fiber substance P in patients
with patellofemoral syndrome.
DISCUSSION
The works of Deising et al. (2012) and Schilder et al.
(2014) clearly demonstrate that the deep fascia has a
different pattern of pain irradiation from the overlying
4 Stecco et al.
skin and underlying muscles: consequently, we must
examine it separately. The organization of the fascia
shows that the deep part is more closely related to
muscles than to skin, both because there is a very large
gliding plane between the superficial and deep fasciae,
which guarantees considerable autonomy between the
structures, and because the deep fascia is tensioned by
myofascial expansions originating from the underlying
muscles. The classification of somatic pain (Byers
and Bonica, 2001; Coda and Bonica, 2001; Pasero
et al., 1999) also combines pain related to the deep fascia
with muscular pain (deep somatic pain), whereas
the skin is related to superficial somatic pain. Consequently,
the innervation patterns of the deep fascia
probably follow myotomes rather than dermatomes.
The anatomical organization of the deep fascia can also
create a massive irradiation of pain along the fascial
reinforcements, which follow a different pattern from
the skin. According to this distinction, it is easy to
explain the “anomalous” pattern of pain irradiation that
does not follow a specific dermatome. For example,
Furman and Stephen (2019) showed that during lumbosacral
transforaminal epidural needle placement and
injections, the distribution of symptoms often differs
from that predicted by classic lumbosacral dermatomal
maps. Slipman et al. (1998) also reported a discrepancy
between radicular pain patterns and classic dermatome
maps in the cervical spine. Murphy et al.
(2009) concluded that in most cases nerve root pain
should not be expected to follow a specific dermatome
and that dermatomal distribution of pain is not a useful
historical consideration for diagnosing radiculopathy.
Kurosawa et al. (2015) and Murakami et al. (2017)
reported that leg symptoms associated with sacroiliac
joint disorder do not usually correspond to the dermatome
(Kurosawa et al., 2015; Murakami et al., 2017).
Bearing in mind the innervation of the deep fascia, the
above authors attribute input to both skin and deep
fascia during nerve root stimulation, creating an overlap
of maps. In fact, maps of the skin and deep fascia
probably overlap in the trunk, where the metameric
organization of the muscles is maintained, but could
diverge considerably in the limbs. In the latter, skin
innervation follows the cutaneous nerves, whereas
muscles show an entirely different pattern. When the
nerve root is examined, not the motor nerves, all the
muscles that stretch the deep fascia of the posterior
compartment are clearly innervated by the same nerve
root—in particular, from S1 in the lower limbs and C7 in
the upper limbs (Furman and Stephen, 2019; Ladak
et al., 2014; Schirmer et al., 2011; Slipman et al.,
1998; Standring, 2016). Similarly, all the muscles
stretching the deep fascia of the anterior compartments
are innervated by L4 and C5; the muscles in the lateral
region of the upper limbs are innervated by C6, and
those in the lower limbs by L5. Lastly, the muscles in
the medial region of the lower limbs are innervated by
L3 and those of the upper limbs by C8 (Fig. 3).
Nerve root stimulation causes muscular contraction,
allowing the bone to move, but it also stretches
the overlying deep fascia thanks to myofascial expansions.
As such, expansions are located along the main
spatial directions, we suggest that all the fascial
receptors along that line are stimulated during a
movement in one direction, and consequently all
these inputs coming from one line converge on a specific
root. In this way, signals are pre-coded in the
periphery, linking the perception of each motor direction
to stretching of the receptors of a precise line of
force inside the deep fascia. Most interestingly, pain
referred to the buttocks, posterior thigh, or posterior
calf cannot be due to radicular compression, but to
excessive tension of the deep fascia along a specific
line of force. This tension can activate all the free
nerve endings along that line, giving rise to pain that
simulates, for example, “S1 radiculitis.” This could
Fig. 3. Clockwise organization of nerve root distribution in limbs. The only difference
between upper and inferior limbs is that adduction is perceived by distal nerve roots in the
former, and by more proximal ones in the latter. This is probably due to limb rotation during
embryological evolution, passing from quadrupedal to bipedal position. (a): movement
line: anteposition (an) or flexion; retroposition (re) or extension; medioposition
(me) or adduction; lateropostion (la) or abduction. (b): nerve root distribution in upper
limb. (c): nerve root distribution in lower limb.
Dermatome and Fasciatome 5
explain the symptoms of patients with predicted “S1
radiculitis” without imaging, supporting S1 pathology.
CONCLUSIONS
Knowledge of the rich innervation of the deep fascia
and its anatomical organization indicates the need to
reevaluate dermatome maps in the light of the new
findings. Only the distinction between dermatome (the
portion of tissue composed of skin, hypodermis, and
superficial fascia supplied by all the cutaneous branches
of a single spinal nerve) and the fasciatome (the portion
of deep fascia supplied by the same nervous root and
organized along the force lines for the four main directions
of movement) can explain the main differences
among dermatomeric maps reported in the literature.
The dermatome is important for esteroception, whereas
the fasciatome follows the precise patterns created
from the deep fascia, which is important for proprioception.
If altered, the dermatome gives clearly localized
pain and the fasciatome irradiating pain, in accordance
with the organization of the fascial anatomy.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Byers M, Bonica JJ. 2001. Peripheral pain mechanisms and nociceptor
plasticity. In: Loeser JD, Butler SH, Chapman CR, et al., editors.
Bonica’s Management of Pain. 3rd Ed. Baltimore, MD: LippincottWilliams
& Wilkins. p 26–72.
Challoumas D, Ferro A, Walker A, Brassett C. 2018. Observations on
the inconsistency of dermatome maps and its effect on knowledge
and confidence in clinical students. Clin Anat 31:293–300.
Coda BA, Bonica JJ. 2001. General considerations of acute pain. In:
Loeser JD, Butler SH, Chapman CR, et al., editors. Bonica’s Management
of Pain. 3rd Ed. Baltimore, MD: Lippincott Williams &
Wilkins. p 222–240.
Deising S, Weinkauf B, Blunk J, Obreja O, Schmelz M, Rukwied R.
2012. NGF-evoked sensitization of muscle fascia nociceptors in
humans. Pain 153:1673–1679.
Denny-Brown D, Kirk E. 1968. Hyperesthesia from spinal and root
lesions. Trans Am Neurol Assoc 93:116–120.
Denny-Brown D, Kirk EJ, Yanagisawa N. 1973. The tract of Lissauer
in relation to sensory transmission in the dorsal horn of spinal
cord in the macaque monkey. J Comp Neurol 151:175–200.
Foerster O. 1933. The dermatones in man. Brain 56:1–39.
Furman MB, Stephen CJ. 2019. Induced lumbosacral radicular symptom
referral patterns: A descriptive study. Spine J 19:163–170.
Gibson W, Arendt-Nielsen L, Taguchi T, Mizumura K, Gravensen T.
2009. Increased pain from muscle fascia following eccentric exercise:
Animal and human findings. Exp Brain Res 194:299–308.
Head H. 1893. On disturbances of sensation with especial reference
to the pain of visceral disease. Brain 16:1–133.
Herringham W. 1886. The minute anatomy of the brachial plexus.
Proc R Acad 41:423–441.
Hoheisel U, Taguchi T, Treede RD, Mense S. 2011. Nociceptive input
from the rat thoracolumbar fascia to lumbar dorsal horn neurones.
Eur J Pain 15:810–815.
Hoheisel U, Rosner J, Mense S. 2015. Innervation changes induced
by inflammation of the rat thoracolumbar fascia. Neuroscience 6:
351–359.
Inman V, Saunders J. 1944. Referred pain from skeletal structures.
J Nerv Ment Dis 99:660–667.
Itoh K, Okada K, Kawakita K. 2004. A proposed experimental model
of myofascial trigger points in human muscle after slow eccentric
exercise. Acupunct Med 22:2–12.
Keegan JJ, Garrett FD. 1948. The segmental distribution of
the cutaneous nerves in the limbs of man. Anat Rec 102:
409–437.
Kirk EJ, Denny-Brown D. 1970. Functional variation in dermatomes in
the macaque monkey following dorsal root lesions. J Comp Neurol
139:307–320.
Kishner S, McMyne RC, Comeaux JA. 2017. Dermatomes anatomy.
Available at: https://emedicine.medscape.com/article/1878388-
overview.
Kurosawa D, Murakami E, Aizawa T. 2015. Referred pain location depends
on the affected section of the sacroiliac joint. Eur Spine J 24:
521–527.
Ladak A, Tubbs RS, Spinner RJ. 2014. Mapping sensory nerve communications
between peripheral nerve territories. Clin Anat 27:
681–690.
Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De Caro R.
2011. Layers of the abdominal wall: Anatomical investigation of
subcutaneous tissue and superficial fascia. Surg Radiol Anat 33:
835–842.
Lee MW, McPhee RW, Stringer MD. 2008. An evidence-based approach
to human dermatomes. Clin Anat 21:363–373.
Marshall R. 2001. Living Anatomy: Structure as the Mirror of Function.
Melbourne: Melbourne University Press. p 222.
Murakami E, Aizawa T, Kurosawa D, Noguchi K. 2017. Leg sympotms
associated with sacroiliac joint disorder and related pain. Clin
Neurol Neurosurg 157:55–58.
Murphy DR, Hurwitz EL, Gerrard JK, Clary R. 2009. Pain patterns
and descriptions in patients with radicular pain: Does the pain
necessarily follow a specific dermatome? Chiropratic Osteopathy
17:9.
Nash LG, Phillips MN, Nicholson H, Barnett R, Zhang M. 2004. Skin
ligaments: Regional distribution and variation in morphology. Clin
Anat 17:287–293.
Pasero C, Paice JA, McCaffery M. 1999. Basic mechanisms underlying
the causes and effects of pain. In: McCaffery M, Pasero C,
editors. Pain Clinical Manual. 2nd Ed. St. Louis, MO: Mosby Inc.
p 15–34.
Sanchis-Alfonso V, Rosellò-Sastre E. 2000. Immunohistochemical
analysis for neural markers of the lateral retinaculum in patients
with isolated symptomatic patellofemoral malalignment. A neuroanatomic
basis for anterior knee pain in the active young patient.
Am J Sports Med 28:725–731.
Schilder A, Hoheisel U, Magerl W, Benrath J, Klein T, Treede RD.
2014. Sensory findings after stimulation of the thoracolumbar
fascia with hypertonic saline suggest its contribution to low back
pain. Pain 155:222–231.
Schilder A, Magerl W, Klein T, Treede RD. 2018. Assessment of pain
quality reveals distinct differences between nociceptive innervation
of low back fascia and muscle in humans. Pain Rep 30:
e662.
Schirmer CM, Shils JL, Arle JE, Cosgrove GR, Dempsey PK,
Tarlov E, Kim S, Martin CJ, Feltz C, Moul M, Magge S. 2011.
Heuristic map of myotomal innervation in humans using direct
intraoperative nerve root stimulation. J Neurosurg Spine 15:
64–70.
Sherrington C. 1898. Experiments in examination of the peripheral
distribution of the fibers of the posterior roots of some spinal
nerves II. Philos Trans R Soc London Ser B 190:45–186.
Slipman CW, Plastaras CT, Palmitier RA, Huston CW, Sterenfeld EB.
1998. Symptom provocation of fluoroscopically guided cervical
nerve root stimulation. Are dynatomal maps identical to dermatomal
maps. Spine 23:2235–2242.
Standring S. 2016. Gray’s Anatomy: The Anatomical Basis of Clinical
Practice. 41st Ed. New York: Elsevier Churchill Livingstone.
6 Stecco et al.
Stecco C. 2015. Functional Atlas of the Human Fascial System. UK:
Elsevier Health Sciences.
Stecco C, Gagey O, Belloni A, Pozzuoli A, Porzionato A, Macchi V,
Aldegheri R, De Caro R, Delmas V. 2007. Anatomy of the deep
fascia of the upper limb. Second part: Study of innervation.
Morphologie 91:38–43.
Stecco C, Porzionato A, Macchi V, Stecco A, Vigato E, Parenti A,
Delmas V, Aldegheri R, De Caro R. 2008. The expansions of the
pectoral girdle muscles onto the brachial fascia: Morphological
aspects and spatial disposition. Cells Tissues Organs 188:320–329.
Taguchi T, Hoheisel U, Mense S. 2008. Dorsal horn neurons having
input from low back structures in rats. Pain 138:119–129.
Taguchi T, Yasui M, Kubo A, Abe M, Kiyama H, Yamanaka A,
Mizumura K. 2013. Nociception originating from the crural fascia
in rats. Pain 154:1103–1114.
Tesarz J, Hoheisel U, Wiedenhofer B, Mense S. 2011. Sensory innervation
of the thoracolumbar fascia in rats and humans. Neuroscience
194:302–308.
Wilke J, Niederer D, Vogt L, Banzer W. 2016. Remote effects of lower
limb stretching: preliminary evidence for myofascial connectivity?
J Sports Sci 34:2145–2148.
Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. 2012.
The thoracolumbar fascia: Anatomy, function and clinical considerations.
J Anat 221:507–536.